Key Points
- Lecanemab (Leqembi®) has been approved by the U. S. Food and Drug Administration (FDA) as a treatment for early Alzheimer’s,
- It is the first approved drug that changes the course of the disease for people in the early stages by addressing the underlying physical causes,
- It is not a cure but slows the disease’s progression to give people more time to live more fully and independently.
What is Alzheimer’s Disease
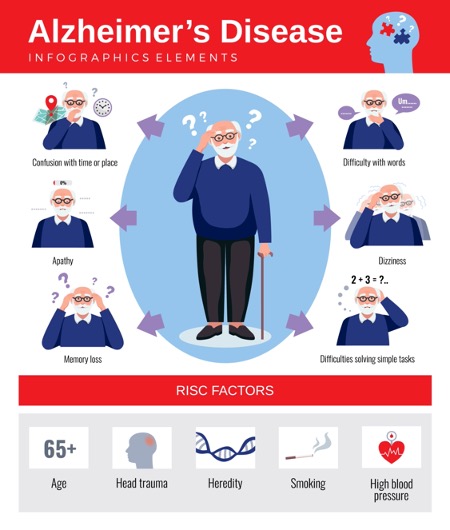
Alzheimer’s is the most common type of dementia. Dementia is a general term for the impaired ability to remember, think or make decisions serious enough to interfere with daily life. Alzheimer’s disease accounts for 60-80% of dementia cases.
- More than 6 million Americans live with Alzheimer’s disease,
- Alzheimer is the fifth leading cause of death for adults over 65,
What causes Alzheimer’s Disease?
It is believed that proteins, called amyloid plaques, form in the brains of people who have Alzheimer’s. These plaques can build up and lead to problems with brain function.
Amyloid plaque is produced by everyone; however, some people can flush out the plaque on a daily basis to avoid buildup. Some experts believe that diet and lifestyle affect this process.
Is this treatment for everyone with Alzheimer’s?
No, the FDA specifies that Lecanemab is only for people with early Alzheimer’s who have been confirmed of elevated beta-amyloid levels.
This therapy has not been tested on people with more advanced stages of Alzheimer’s. It has also not been tested on those who do not have symptoms.
Is there a test to check for Alzheimer’s?
No, there is no single diagnostic test to determine if a person has Alzheimer’s disease. Doctors use a variety of ways to help make a diagnosis.
To diagnose Alzheimer’s, they may use any of the following:
- Medical history
- Mental status tests
- Physical and neurological exams,
- Biofluid (CSF and blood) tests
- Brain imaging.
Before starting this treatment, a doctor must confirm the presence of beta-amyloid plaques.
How expensive is Leqembi?
Eisai and Biogen, the manufacturers of lecanemab, announced they are setting the price of the drug at $26,500 a year.
The Centers for Medicare & Medicaid Services (CMS) announced they will cover lecanemab if a patient’s doctor enrolls them in a CMS-run registry.
Will Medicaid cover it?
Those eligible for both Medicare and Medicaid — either as a Full Benefit Dual Eligible (eligible for full Medicaid benefits) or in a Medicare Savings Program (Medicaid helping to cover some of the beneficiary’s Medicare costs) can receive coverage.
There may be additional state requirements for those eligible for both Medicaid and Medicare.
How is this drug administered?
The treatment is given every two weeks through an IV, lasting about one hour for each infusion. Usually, infusions can be done at hospitals and infusion therapy centers.
What are the side effects?
As with other similar anti-amyloid treatments, Lecanemab does have side effects, including serious allergic reactions. The most common reported side effects were reactions to the infusion and imaging processes and headache.
Ivan Cheung, the Americas region chairman and CEO of Tokyo, Japan-based Eisai, said the FDA approval of Leqembi is a “triumph for the Alzheimer’s disease community” because it’s the first medication to slow the progression of the disease. “With Leqembi, patients can get more time.” Cheung told USA TODAY.
Images by kjpargeter and by macrovector on Freepik
Thank you, You will be automatically subscribed to the our newsletter.